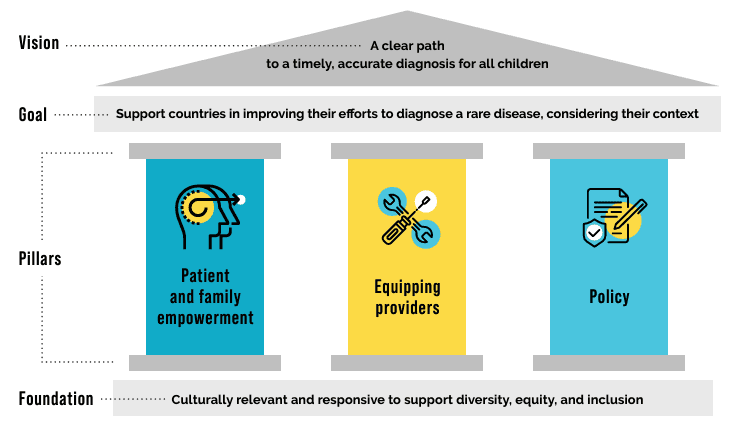
In May 2024 the Global Commission announced the first iteration of a framework to support global, regional, and national efforts to end the diagnostic odyssey.
To bring the framework to life, the Global Commission is assembling a compendium of case studies with the intent to showcase innovative efforts to accelerate diagnosis.
We aim to continue to add to this resource compendium over time. Please check back regularly for new updates or submit a case study for consideration at any time.

Find a Case Study
























The Global Commission to End the Diagnostic Odyssey for Children with a Rare Disease is an association composed of a diverse group of organizations and individuals committed to shortening the time for an accurate diagnosis for all children with rare diseases. The views expressed herein are solely those of the Commission and do not represent any individual, co-chair, or member organization’s views or opinions.